Detailed Picture of Protein Complex Reveals Potential Therapeutic Target for Lupus, Study Reports
Written by |
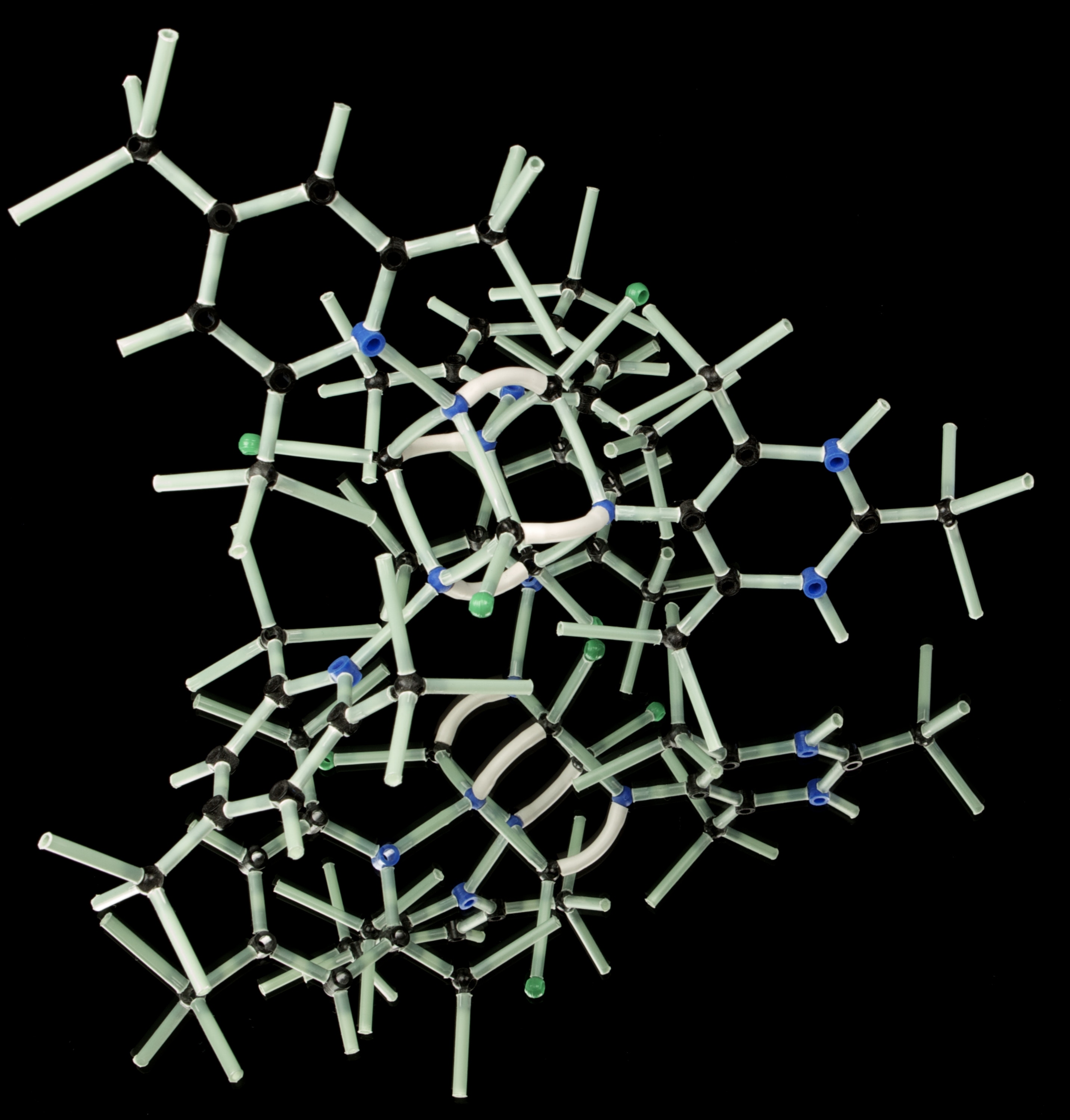
Scientists have taken a high-resolution photo of a protein complex, called BRISC-SHMT2, that promotes inflammation and is thought to be at the root of lupus symptoms.
This factor may represent a new therapeutic target to tackle the disease, and the new findings may help researchers find therapies that disrupt this molecule and prevent the self-attacking immune responses underlying lupus.
The study, “Metabolic control of BRISC–SHMT2 assembly regulates immune signalling,” was led by researchers at the University of Pennsylvania and University of Leeds and was partially funded by the Lupus Research Alliance. It was published in the journal Nature.
The scientists were interested in studying a protein complex made up of two major “pieces,” namely the SHMT2 protein and a cluster of proteins called BRISC.
When these two pieces bind to each other and form the SHMT2-BRISC complex, they normally trigger inflammation in response to invading harmful microorganisms.
However, previous research from the lab of Roger Greenberg, MD, PhD, from the University of Pennsylvania, and senior co-leader of the study, demonstrated that if mice lack BRISC, they stop responding to type I interferons, a type of immune signaling molecule, or cytokine, implicated in lupus.
In fact, Greenberg and his team saw that mice deficient for BRISC were less likely to develop lupus.
Patients with lupus often produce too many type I interferons, which overactivate certain genes involved in inflammation, causing lupus symptoms.
Greenberg won a Target Identification in Lupus (TIL) grant from the Lupus Research Alliance in 2016 to study if molecules that block BRISC are able to alleviate lupus symptoms in mice.
Given the apparent role of SHMT2-BRISC interaction for inflammation and disease, the researchers were interested in understanding more about how these two pieces bind to each other. For this purpose, they used cryo-electron microscopy, a technique that enabled them to take high-resolution pictures at the atomic level of the protein complex.
They found that four copies of BRISC combine to form a U-shaped structure that is strengthened by crossbeams of SHMT2, and they discovered that small changes to the shape of BRISC broke up its pairing with SHMT2.
Once the structure of the pair had been revealed in detail, it was possible to design genetically engineered versions of the proteins to see what role they played in cells.
Mutations in BRISC that prevented it from binding SHMT2 inhibited many genes involved in type I interferon signaling from working properly, following an inflammatory stimuli. This suggested that a compound able to stop the BRISC and SHMT2 pairing may provide a new way to treat lupus symptoms.
“We want to find a drug targeting BRISC to decrease interferon and other inflammatory signals so we can help lupus patients,” Greenberg said in a press release. “Knowing the weakness in the BRISC-SHMT2 structure gives us new targets to work with.”
The scientists were also surprised to see that the BRISC-SHMT2 complex can be regulated by the active form of vitamin B6. Even though they caution that more research is needed to fully understand the role of this vitamin, this discovery gave them “crucial insights on the role of metabolism in regulating innate immunity,” Greenberg said in a University of Leeds press release.
“The microscopes have allowed us to understand the structure of this protein complex in superb detail, and by building an accurate 3D model, we discovered that it plays a completely unexpected role in regulating our immune response.” said Elton Zeqiraj, PhD, from the University of Leeds and one of the study’s senior authors.
“We’re a long way off being able to find a new effective treatment for autoimmune disease, but we’re excited because this discovery could open the door to a new class of drugs,” he added.
“Dr. Greenberg’s results are very exciting, pointing to potential targets for new drugs to be developed. We saw great potential in his TIL proposal and look forward to further progress in his work,” said Kenneth M. Farber, Lupus Research Alliance president and CEO.




