Benlysta (belimumab) for lupus
Last updated June 21, 2024, by Marisa Wexler, MS

What is Benlysta for lupus?
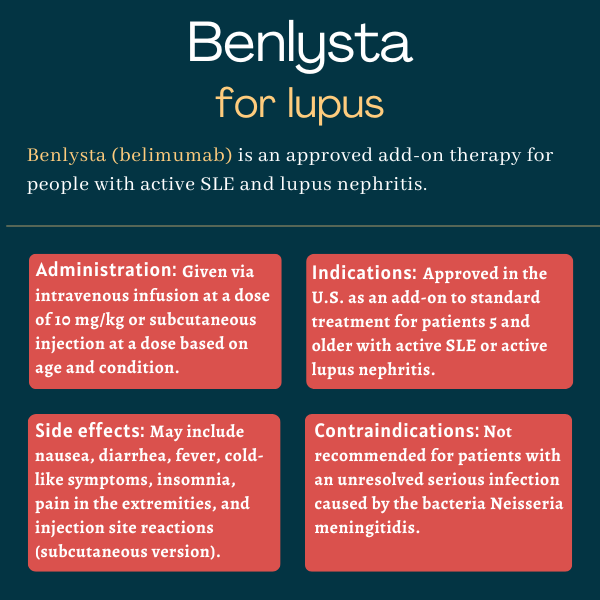
Benlysta (belimumab) is a monoclonal antibody approved to help treat people with active systemic lupus erythematosus (SLE) or active lupus nephritis who are receiving standard treatment.
It can be administered via an into-the-vein (intravenous) infusion or subcutaneous (under-the-skin) injection.
The therapy is marketed by GSK, formerly known as GlaxoSmithKline.
Therapy snapshot
| Brand name: | Benlysta |
| Chemical name: | Belimumab |
| Usage: | Add-on to standard therapy for active SLE and active lupus nephritis |
| Administration: | Intravenous infusion or subcutaneous injection |
How does Benlysta work?
B-cells are a type of immune cell responsible for producing antibodies, which help the body fight off infections by sticking to viruses and bacteria and directing an immune attack against the infectious invader. In lupus, B-cells produce antibodies, referred to as autoantibodies, that direct an attack against the body’s own healthy cells.
Benlysta is an antibody-based therapy designed to reduce the production of disease-driving autoantibodies by lowering B-cell activity and survival. The therapy doesn’t bind to B-cells directly. Instead, it binds to a protein in the blood called B-lymphocyte stimulator (BLyS), also referred to as B-cell activating factor (BAFF). Normally, BLyS in the blood (referred to as “soluble” BLyS) interacts with receptors on B-cells to promote their survival and activation. By preventing BLyS from interacting with those receptors, Benlysta inhibits the survival of B-cells, including the ones that produce autoantibodies, and reduces B-cell differentiation into plasma cells, which can produce large amounts of antibodies within a short period of time.
According to GSK, Benlysta is the only approved medication in the U.S. that’s designed to target BLyS/BAFF, the underlying cause of lupus and lupus nephritis.
Who can take Benlysta?
Benlysta is approved in the U.S. for patients 5 and older who have SLE, the most common form of lupus, or lupus nephritis, a potentially dangerous complication of lupus marked by kidney damage and inflammation. The therapy is indicated for use as an add-on treatment in patients who are receiving standard therapy.
Benlysta was originally approved by the U.S. Food and Drug Administration (FDA) in March 2011 for adults with active SLE, becoming the first new medication to be approved for SLE in more than 50 years. In December 2020, its approval was extended to adults with active lupus nephritis. Benlysta was approved for children 5 and older with SLE in April 2019 and to treat lupus nephritis in this younger patient population in July 2022. The FDA authorized an under-the-skin formulation of Benlysta for children with SLE in May 2024, marking the first time children with the condition could receive the therapy at home.
Benlysta is currently the first and only biological therapy to be approved in the U.S. for both SLE and lupus nephritis in children and adults in more than five decades.
The therapy has also been approved for similar indications in Europe, Canada, and other countries.
Who should not take Benlysta?
Benlysta is contraindicated, or not recommended, for anyone who has had an allergic reaction to its active ingredient belimumab. The medication has not been evaluated in people with severe active central nervous system lupus and therefore not recommended for people with this complication.
Data from clinical trials suggest Black patients may be less likely to respond to Benlysta than people of other racial backgrounds. Patients are advised to talk about the potential risks and benefits of the therapy with their healthcare teams.
How is Benlysta administered?
Benlysta can be administered via an intravenous infusion or subcutaneous injection.
The infusion version comes in single-dose vials containing 120 or 400 mg of the therapy in a white to off-white powder form, which should be diluted in 1.5 or 4.8 mL of sterile water, respectively, before being administered. The process of preparing the therapy for infusion is carried out by a medical professional.
The subcutaneous version of Benlysta comes as a single-dose prefilled autoinjector or a single-dose prefilled glass syringe containing 200 mg/mL of belimumab in a clear to opalescent and colorless to pale yellow liquid. The injection may be given into the skin of the abdomen or thigh, and with proper training, it can be performed at home by patients or caregivers. For children younger than 10, the medication should be administered by a healthcare professional or a trained caregiver.
The approved intravenous dosage for adults and children with SLE or lupus nephritis is 10 mg/kg of body weight, given at two-week intervals for the first three doses, and at four-week intervals thereafter. Each infusion takes about an hour, and it’s recommended that patients receive preventive medication before each infusion to decrease the risk of infusion or allergic reactions.
For the subcutaneous formulation, dosage depends on the patient’s age and condition. In adults with SLE, the approved dosage is 200 mg once weekly. This dosage is also used in children with SLE who weigh at least 40 kg (about 88 lbs), whereas for children who weigh less than 40 kg, the approved dose is 200 mg every other week.
In adults with lupus nephritis, the approved dose is 400 mg (requiring two autoinjectors or syringes) once weekly for the first four weeks, followed by 200 mg once weekly thereafter. The safety and efficacy of subcutaneous Benlysta in children with lupus nephritis has not been established.
Benlysta in clinical trials
The approvals of the different formulations of Benlysta in adults and children with active SLE and active lupus nephritis were supported by a number of clinical trials.
Intravenous Benlysta in adults with SLE
The initial approval of Benlysta was supported by data from a pair of Phase 3 trials — BLISS-76 (NCT00410384) and BLISS-52 (NCT00424476) — that collectively enrolled more than 1,600 adults with SLE at sites in North and South America, Asia, Europe, and Australia. The design of both trials was based on data from a previous Phase 2 trial (NCT00071487) which indicated that Benlysta was beneficial for patients who test positive for autoantibodies.
Participants were randomly assigned to receive Benlysta at one of two doses (1 or 10 mg/kg) or a placebo for at least 52 weeks (one year), in addition to standard therapy.
All the participants were positive for autoantibodies and more than 70% in both trials were taking at least two other lupus medications, including corticosteroids, antimalarial agents, and/or immunosuppressants.
In both studies, the main goal was to determine the number of patients who met the SLE Responder Index-4 (SRI-4) criteria after a year. SRI-4 is a composite measure of disease severity that basically means patients have improved in some measures of disease activity, without worsening in others.
In both trials, the proportion of patients achieving SRI-4 was significantly higher among those treated with 10 mg/kg of Benlysta than in those given a placebo (43% vs. 34% in BLISS-76; 58% vs. 44% in BLISS-52). The lower dose also showed positive trends in SRI-4 rates, but the results were statistically significant only in BLISS-52.
In both trials, fewer patients on 10 mg/kg of Benlysta than on a placebo had a severe disease flare (a sudden worsening of symptoms).
An exploratory analysis of 148 Black participants across both studies showed that, in this racial group, the SRI-4 response rate was higher among those given a placebo than in those on Benlysta.
This prompted the launch of the Phase 4 EMBRACE study (NCT01632241) that compared the safety and efficacy of 10 mg/kg of Benlysta against a placebo, given on top of standard therapy in 448 Black SLE patients. The trial had a similar design to the earlier studies and again the goal was to determine the patient response rate through another composite measure of disease activity. Results showed the response rate was higher among patients given Benlysta (49% vs. 42%), though the difference was not considered statistically significant compared with the placebo.
Intravenous Benlysta in pediatric SLE
Benlysta’s approval for children with SLE was supported by data from a Phase 2 trial called PLUTO (NCT01649765) that enrolled 93 children with SLE. Participants were randomly assigned to receive 10 mg/kg of Benlysta or a placebo, on top of standard therapy, for a year. Like the initial studies of SLE in adults, the main goal of PLUTO was to determine the patient responder rate based on SRI-4.
Results showed the responder rate was higher among patients on Benlysta than on a placebo (53% vs. 44%), though the difference between the groups did not reach statistical significance. The proportion of children who had a severe disease flare was lower among those on Benlysta compared with a placebo (17% vs. 35%), reflecting a 64% lower risk of having a severe flare over a year.
Subcutaneous Benlysta in SLE
The safety and effectiveness of Benlysta given by a subcutaneous injection was assessed in a Phase 3 trial called BLISS-SC (NCT01484496) which enrolled 836 adults with SLE. Participants were randomized to be treated with subcutaneous Benlysta (200 mg once weekly) or a placebo, on top of standard therapy, for a year. As in studies assessing the safety and effectiveness of intravenous Benlysta, the main goal of BLISS-SC was to determine patient responder rate based on SRI-4.
Results showed the SRI-4 responder rate was significantly higher for patients on Benlysta than for those given a placebo (61% vs. 48%) after a year. In an exploratory analysis of 91 Black patients, the response rate was slightly higher among those on Benlysta compared with a placebo (45% vs. 39%). Severe disease flares were seen in 11% of patients on Benlysta compared with 18% on a placebo, indicating patients on Benlysta had a 49% lower risk of having at least one severe lupus flare over a year.
Intravenous Benlysta in lupus nephritis
Benlysta’s approval for adults with lupus nephritis was supported by data from a Phase 3 trial called BLISS-LN (NCT01639339) which enrolled 448 adults with this lupus complication at sites in Asia, North America, South America, and Europe. Participants were randomly assigned to receive 10 mg/kg of Benlysta or a placebo, given on top of standard therapies, for two years.
The study’s main goal was to determine the patient response rate based on the Primary Efficacy Renal Response (PERR), a composite measure of kidney function. After two years, the proportion of PERR responders was significantly higher among patients on Benlysta compared with those on a placebo (43% vs. 32%).
A similar difference was seen at one year (47% vs. 35%), and other measures of kidney function generally favored Benlysta. Patients on Benlysta were significantly less likely than those on a placebo to die or experience a serious kidney-related health event.
Common side effects of Benlysta
The most common side effects of Benlysta include:
- nausea
- diarrhea
- fever
- the common cold
- bronchitis (lung inflammation)
- insomnia
- pain in the extremities
- depression
- migraine
- sore throat
- injection site reactions (with subcutaneous administration only).
Serious infection risks
Benlysta works by reducing the activation of the immune system, which normally helps protect the body against infection. Serious and sometimes fatal infections have occurred in people taking Benlysta. The risks and benefits of the medication should be carefully considered before starting treatment in patients with a history of severe or chronic infections. Interrupting treatment should be considered if a new infection develops.
If patients show signs and symptoms of neurological damage, they should be evaluated for a rare form of brain infection called progressive multifocal leukoencephalopathy (PML). Cases of PML, including fatal cases, have been reported in patients on Benlysta. If PML is diagnosed, Benlysta and other immune-suppressing medicines should be discontinued.
Allergic reactions
Allergic reactions to Benlysta, including serious and fatal allergic reactions and infusion-related reactions, have been reported. These reactions usually occur within a few hours after an infusion, but may occur much later.
If patients show signs of a serious allergic reaction, Benlysta should be discontinued immediately and appropriate treatment should be started. In the case of an infusion-related reaction, the therapy’s infusion rate may be slowed or interrupted. It’s also recommended that patients receiving intravenous Benlysta be monitored for an appropriate period of time after receiving an infusion.
Depression and suicidality
Depression and suicidality have been reported in trials of Benlysta. Patients should undergo a mental health evaluation before starting treatment and should be monitored during treatment. Those on Benlysta should contact their healthcare team if they experience new or worsening mood changes.
Cancer risk
Although the use of immune-suppressing therapies has been linked to an increased risk of cancer, the effect of Benlysta treatment on cancer risk is not known. Benlysta’s benefit-risk profile should be carefully considered in patients with known risk factors for cancer development or recurrence. In those who develop cancer, the medication’s benefit-risk profile should be considered when deciding whether or not to continue treatment.
Vaccines and immunization
Because of its mechanism of action, Benlysta may interfere with the body’s response to vaccines. Due to the risk of infection and related complications, vaccines that contain a live virus should not be given to those currently on Benlysta or in the 30 days before starting treatment.
Use with other biological therapies
Studies have indicated an increased incidence of serious infections and body-wide injection reactions in patients receiving Benlysta alongside rituximab, a B-cell depleting therapy sometimes used for lupus. Thus, available data do not support the use of Benlysta with rituximab in SLE patients. The safety and efficacy of Benlysta when used alongside other biological therapies have not been established. The therapy should, therefore, be used with caution when administered in combination with such therapies.
Use in pregnancy and breastfeeding
Not enough data are available about how Benlysta affects pregnancy to determine the risk of miscarriage or complications for the fetus. Data from animals have generally shown no toxicity for the fetus when Benlysta is used during pregnancy, though some data suggest treatment may affect immune system development.
A registry monitors patient outcomes when Benlysta is used during pregnancy. Clinicians can register their pregnant patients by calling 1-877-311-8972 or visiting the website.
There is no data on the use of Benlysta while breastfeeding. The benefits and potential risks of using or stopping Benlysta during pregnancy or breastfeeding should be discussed between the patient and their healthcare team.
Lupus News Today is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- Does my inability to work mean I am disabled or differently abled?
- Living with lupus entails a never-ending learning curve
- Dapirolizumab pegol eases SLE disease activity in Phase 3 trial
- The TARDIS inside us: A metaphor for living with lupus
- How to forge your own advocacy path, no matter where you’re at



