FDA Approves Self-Injectable Formulation of Benlysta for SLE Patients
Written by |
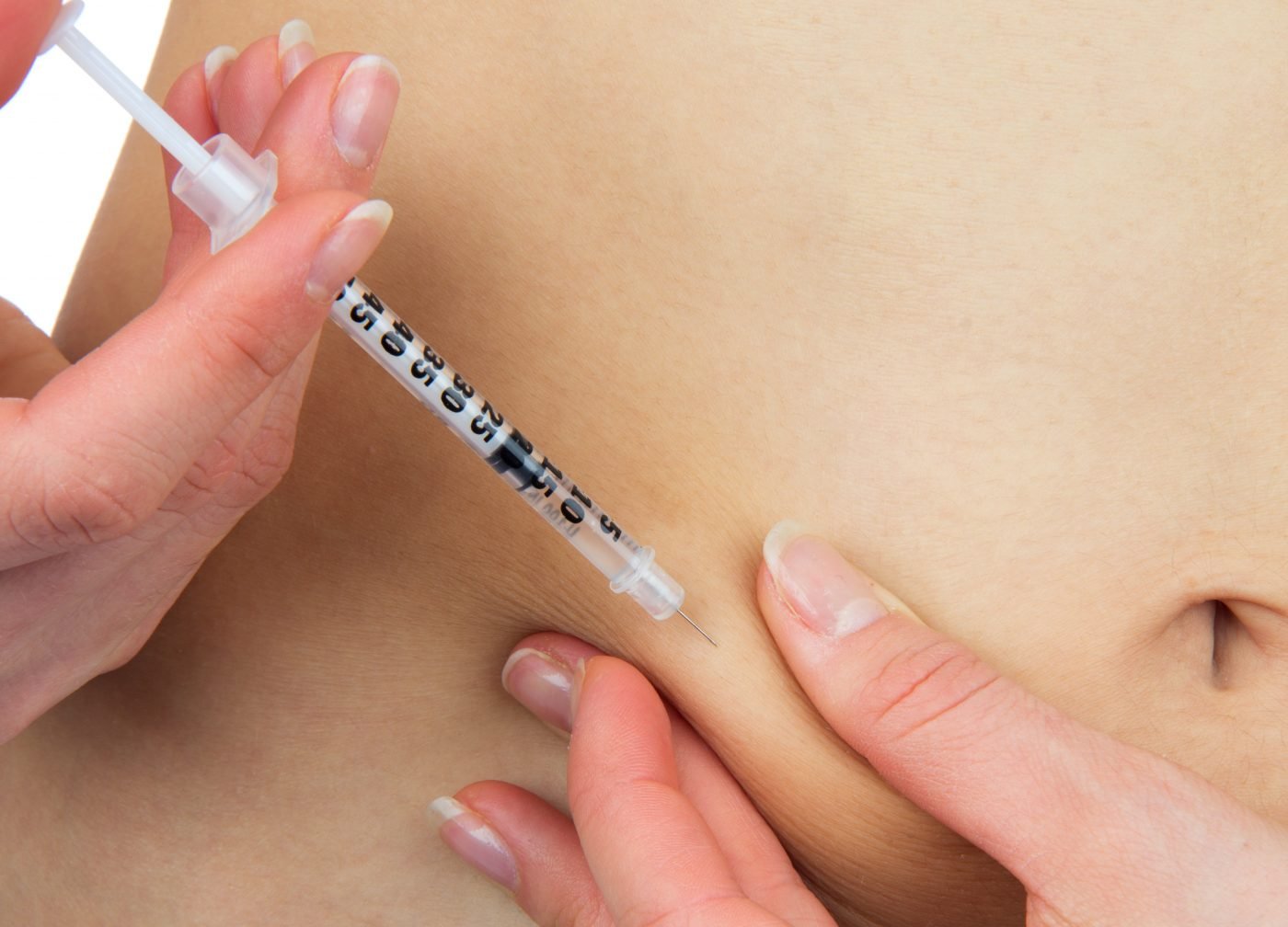
The US Food and Drug Administration (FDA) has approved GSK’s new Benlysta subcutaneous formulation. With the approval, Systemic Lupus Erythematosus (SLE) patients will now be able self-inject Benlysta once weekly.
In 2011, the FDA approved a formulation of Benlysta that was injectable by healthcare professionals to patients as a weight-based dose of 10mg/kg. This treatment required that patients go to a hospital or clinic where they would receive Benlysta during one-hour of intravenous infusion. Following an initial treatment phase on days 0, 14, and 28, patients receive a dose every four weeks.
Now, with this new formulation, patients may choose to self-administer Benlysta as a once weekly injection of 200mg, from either a single-dose prefilled syringe or from a single-dose auto-injector. Subcutaneous Benlysta will be available in specialty pharmacies in the US in late August.
Benlysta (belimumab) is a human monoclonal antibody that prevents auto-reactive immune B cells in SLE patients. It binds to the B-cell activating factor (BAFF), also known as B-lymphocyte stimulator (BLyS), inhibiting B cell survival.
“We are delighted with today’s decision. Lupus can impact the lives of patients in many different ways with varied and often unpredictable symptoms,” Vlad Hogenhuis, Senior Vice President, Head of Specialty Care, GSK said in a press release. “Since it launched in its IV form, thousands of patients worldwide have received treatment with Benlysta. The approval of the new injectable formulation will now provide an additional choice for patients, allowing them to self-administer their medicine at home rather than going to hospitals or clinics for their infusions.”
The approval for the subcutaneous Benlysta formulation followed a clinical trial (ClinicalTrials.gov, number NCT01484496) with more than 800 SLE patients. For 52 weeks, patients received Benlysta together with standard therapy (corticosteroids, antimalarials or non-steroidal anti-inflammatory drugs [NSAIDs]). The study evaluated disease activity based on SLE Responder Index and compared this with patients who received placebo plus standard therapy.
Although Benlysta is indicated for some SLE patients, its efficacy has to be addressed in patients with severe active lupus nephritis or severe active central nervous system lupus. Also, Benlysta’s effects in combination with other biologics or intravenous cyclophosphamide require further studies. Thus, Benlysta is not yet recommended in these situations.




