Lupus Can be Controlled Maintaining Normal Immune Reactions, Study Finds
A study from the Scripps Research Institute shows that harmful autoimmune responses, as seen in diseases like systemic lupus erythematosus (SLE), can be controlled without compromising the normal activity of the immune system when fighting viruses and bacteria. The key, according to the study, is the membrane receptor sphingosine 1-phosphate receptor 1 (S1PR1).
The study, “S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-α autoamplification,” appeared online in the journal Proceedings of the National Academy of Sciences.
The La Jolla, California-based Scripps team, led by Hugh Rosen, had for years been investigating S1PR1 as a potential treatment for autoimmune diseases. Eight years ago, they found an S1PR1 receptor agonist that could block inflammation. Based on this knowledge, Celgene developed the drug ozanimod, currently in Phase 3 clinical trials for multiple sclerosis and ulcerative colitis.
In the first clinical trials, the research team noticed that in some patients, they could see a treatment response also following low doses. The researchers figured that an additional unknown mechanism could explain the patients’ response.
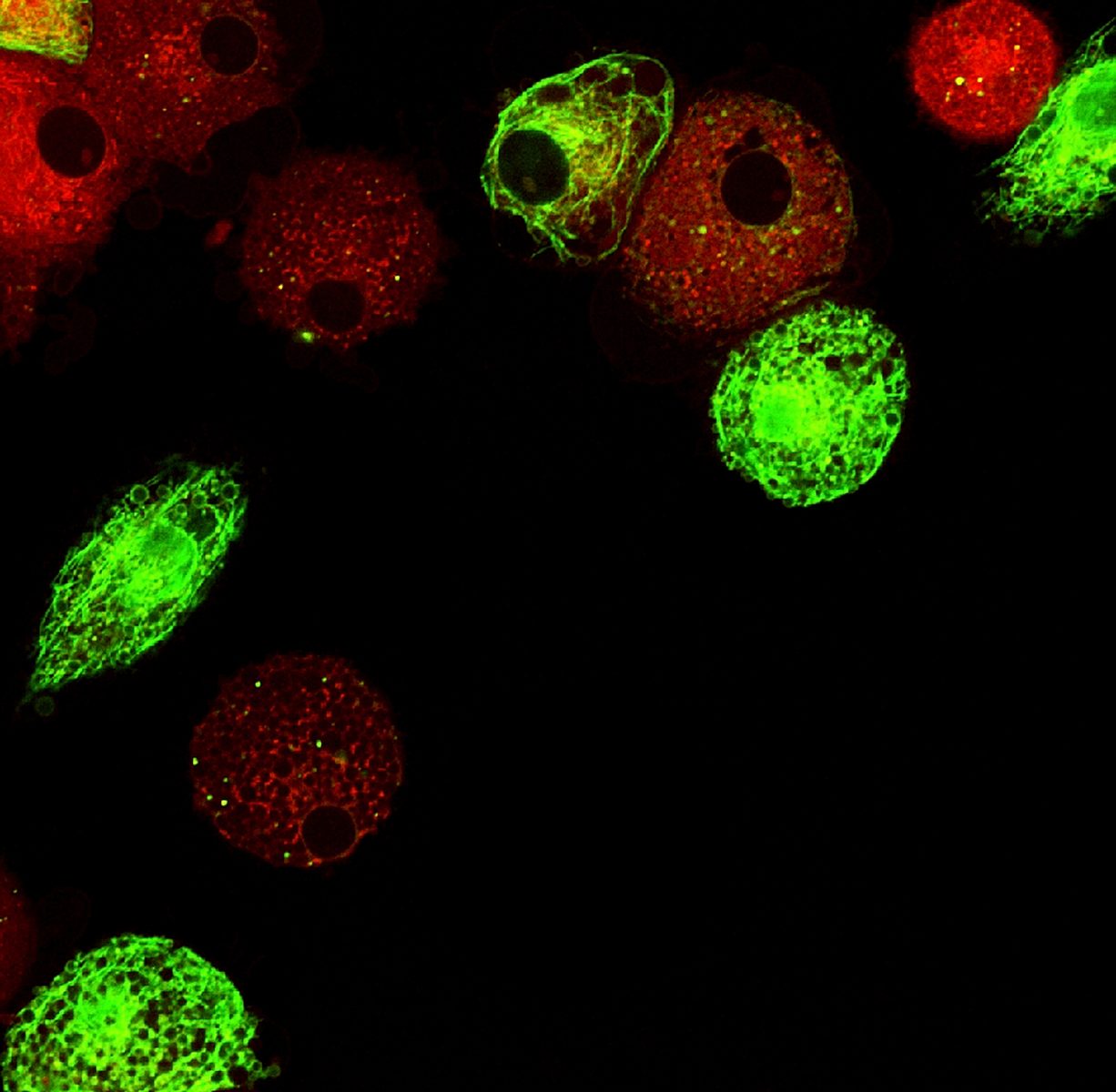
By studying isolated cells from both mice and humans, the team found that S1PR1 agonists target immune cells called plasmacytoid dendritic cells (pDCs). This causes a decrease in the overproduction of factors known to cause autoimmune disease – type-1 interferons, which are crucial in fighting off infections but in autoimmune diseases an auto-amplification loop makes it possible for the immune system to turn on its own signaling.
When agonists stimulate S1PR1 signaling, the S1PR1 receptor is transferred to the inside of the cell and is usually degraded. By treating cells with the S1PR1-targeting compound CYM-5442, upon S1PR1 internalization the type-1 interferon receptor (IFNAR1) is also internalized and degraded. With the interferon receptor degraded, the self-increasing pathway could not be initiated.
“Sphingosine 1-phosphate modulation of the interferon auto-amplification loop by induced turnover of the IFNAR1 is a potential mechanism to limit excessive inflammation physiologically,” Rosen said in a press release. “Augmentation of this mechanism by drugs like ozanimod may allow the successful treatment of patients with difficult-to-treat autoimmune diseases characterized by interferon-a signatures, such as subsets of patients with systemic lupus and rheumatoid arthritis.”
In earlier studies, the Scripps team had noticed that mouse models of autoimmune disease treated with S1PR1 agonists still recognized and reacted to bacteria and viruses. This time, they managed to observe that S1PR1-treated mouse dendritic cells initially released the type-1 interferon in response to a virus, but without triggering the auto-amplification loop.
The researchers state that the potential benefit of drugs such as ozanimod would be to limit tissue damage due to an overactive immune response while maintaining the ability to react to infections caused by microbes – a task today’s drugs are unable to manage. “The findings suggest one could optimize drugs for their ability to suppress this pathway,” Rosen said in the release.